What is the CCES’ position regarding supplement use?
Athletes are responsible for any prohibited substance found in their sample. Unlike foods and medications, the supplement industry is subject to little government regulation making it impossible for the CCES to confirm whether or not a supplement contains prohibited substances. After a number of anti-doping violations related to supplement use, the CCES would like to stress to the Canadian sport community the extreme risk an athlete runs when using supplements.
Under no circumstances can the CCES confirm whether or not a supplement contains prohibited substances.
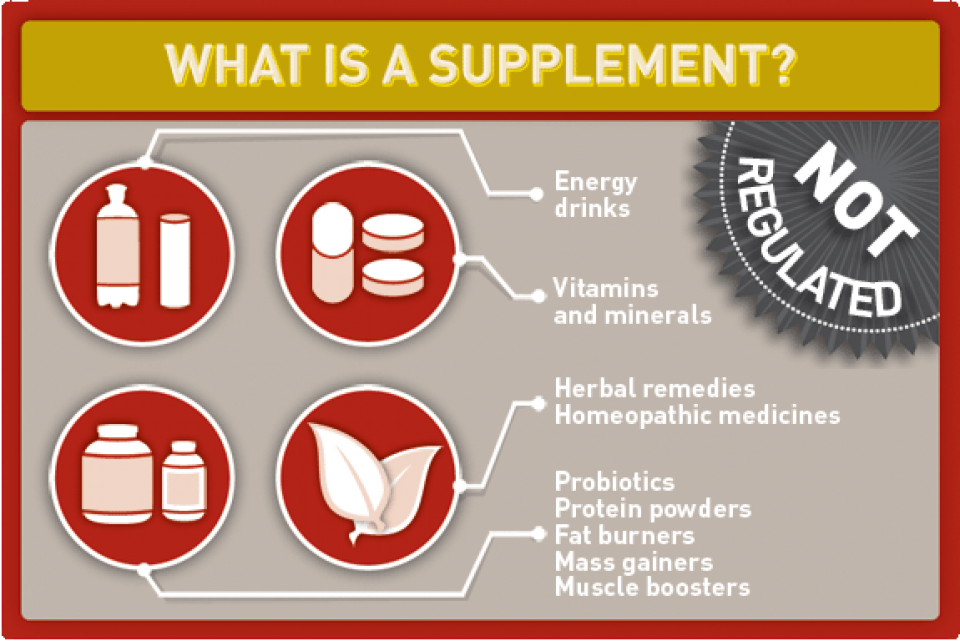
What are supplements?
Supplements are sometimes referred to as nutritional/dietary supplements or natural health products. They are not classified as food or drugs and are not covered by the Canadian Food and Drugs Act.
Supplements include such products as:
- Vitamins and minerals, e.g., calcium, vitamin E
- Some hemp products, e.g., hemp protein, hemp oil
- Herbal remedies, e.g., arnica, echinacea
- Homeopathic medicines, e.g., sulfur, arsenic
- Traditional medicines such as traditional Chinese and Ayurvedic medicines
- Probiotics, e.g., acidophilous, lactobacillus
- Amino acids and essential fatty acids, e.g., glutamine, taurine
- Protein powders, e.g., whey, hemp, soy
- Fat burners and weight-loss products, e.g., Hydroxycut, green coffee bean extract
- Muscle boosters and mass gainers, e.g., Jacked 3D, Hemo-Rage Black Ultra Concentrate
What are the risks associated with supplement use?
Unlike food and pharmaceutical production, the supplement industry is subject to little government regulation. Consequently supplements may:
- Intentionally contain prohibited substances;
- Unintentionally be contaminated with prohibited substances (e.g., contaminated source ingredients, erroneous source ingredients, cross-contamination during manufacturing); or
- Be mislabelled.
In addition, supplements may:
- Not accurately list the ingredients (e.g., falsify, omit);
- Not accurately list the relative amounts of each ingredient per dose;
- Make false certification claims (e.g., WADA-approved);
- Make false health benefit claims; and
- Not list important cautionary information (e.g., side-effects to health).
The number of anti-doping violations resulting from the use of supplements demonstrates the extreme risk an athlete runs when using supplements. A positive test for an athlete who uses supplements may result in a violation regardless of how the prohibited substance got into their body. Serious sanctions may be imposed.
How can athletes minimize the risks?
Athletes have a personal responsibility to evaluate all the risks associated with the consumption of supplements before using them, and are responsible for any prohibited substance found in their sample – this is known as strict liability.
The CCES refers members of the Canadian sport community to the NSF International Certified for Sport® program which helps minimize the risk of unintentional doping. NSF International's Certified for Sport® laboratory testing services help clients establish product stewardship by confirming content and purity, compliance, and assessing public safety and environmental concerns on products used by athletes.
No! For these reasons:
- Some products don’t list all of the ingredients on the label.
- Some products have been adulterated with banned substances (that ARE NOT listed on the label) during the manufacturing or packaging process.
- Some products are made from low-grade ingredients obtained from unreliable sources.
All that to say, checking the ingredient list on a label still doesn’t mean you can be sure about what is actually in the product, and you run the risk of testing positive for a prohibited substance!
If you find supplement ingredients in the Global DRO, the associated status applies only to pharmaceutical-grade ingredients that are included in Health Canada-approved medications. The Global DRO does not contain information on, or that applies to, dietary supplements or natural health products.
As mentioned above, the government does not rigorously regulate the supplement industry. This means that some products may intentionally contain prohibited substances, while others may be inadvertently contaminated with prohibited substances. Because of this, we can never really be sure of what is in these products, and therefore can’t include their status in sport in our resources. The Global DRO exists to help you find the status of medications – the production of which is strictly regulated and monitored.
There is no way of guaranteeing that any supplement or natural health product is completely safe. Ultimately, athletes are responsible for any prohibited substance that may be found in their sample; this is known as strict liability. If athletes who use supplements test positive for a prohibited substance, this can result in a violation, regardless of how the prohibited substance got into their body. Serious sanctions may be imposed.
Unfortunately not. The federal government has few regulations related to the testing of supplements and natural health products, so it’s impossible for the CCES to guarantee that any supplement or natural health product is 100% free of prohibited substances.
Some companies claim that their products are certified by well-known sport organizations like the CCES, the World Anti-Doping Agency (WADA), or the International Olympic Committee (IOC) in an attempt to legitimize their brand. As a smart consumer, you should know that these organizations do not have certification programs for supplements or natural health products. Sketchy labeling is a good reason to second-guess the nature of the product. There is always a risk that these products may contain undisclosed prohibited substances.
Health Canada warns against the following potential risks associated with supplement or natural health product use:
- Manufacturing problems like contamination, incorrect ingredients or dosage suggestions;
- Unproven claims which can lead people to use the wrong products to treat serious conditions or to delay proper treatment;
- Not enough information for people to make an informed choice, like incorrect instructions or no warnings that a product may not be suitable for certain users;
- Interaction with prescription drugs or other natural health products leading to unpredictable side effects or interference with drug action;
- Unwanted side effects, like allergic reactions.
Visit Health Canada's About Natural Health Products web page.